Poster
Update on the preclinical use of hPBMC for immuno-oncology modeling
Contact the preclinical oncology scientific team to learn more.
In February, we presented initial data of Labcorp's emerging humanized mouse platform utilizing human peripheral blood mononuclear cell (hPBMC) engraftment.
Our data using the NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ (NSG) mouse resulted in identification of four hPBMC donors that exhibited a treatment window of at least 30 days post hPBMC injection, consistent T cell engraftment, and growth of two human xenograft models, MiaPaCa-2 (pancreatic) and A549 (NSCLC), that was robust in hPBMC-engrafted NSG mice.
We now present initial efficacy data demonstrating use of hPBMC-engrafted NSG mice bearing MiaPaCa-2 or A549 human xenografts following treatment with pembrolizumab (Keytruda®, anti-hPD-1).
Experimental Design
The objective of these studies was to evaluate the anti-tumor response of pembrolizumab against the MiaPaCa-2 or A549 human tumor models following the engraftment of hPBMCs in NSG mice. Animal care and use was performed in conformance with the Guide for the Care and Use of Laboratory Animals in an AAALAC-accredited facility.
NSG mice (Jackson Laboratories, Bar Harbor Maine, USA, strain #0005557) were intravenously administered hPBMCs from three different normal healthy donors (Hemacare, Los Angeles CA, USA) once tumors became established (~105-118mm3). Treatment with pembrolizumab began the day after hPBMC injection. Response to pembrolizumab, evaluation of onset of Graft vs. Host Disease (GvHD)-like characteristics were determined, and whole blood was collected at two timepoints for flow cytometry analysis of human lymphocyte markers to confirm donor engraftment of CD45+ cells, including CD4+ and CD8+ T cells.
MiaPaCa-2 and A549 Control Tumor Growth in hPBMC Engrafted Mice
MiaPaCa-2 tumor doubling time (Td) in untreated control animals was ~7 days, Td in animals administered hPBMCs ranged from ~5 to 7 days across each donor group.
Tumors grew uniformly to a mean tumor volume of 1000-1200mm3 by day 36 across all three donors, however, some intragroup and intergroup variability was seen beyond day 36. Tumor growth before 1000mm3 did not appear to be impacted by engraftment of hPBMCs (Figure 1).
These results suggest that utilization of any of these three donors would be suitable for MiaPaCa-2 efficacy studies with a time to evaluation size (TES) of 1000mm3.
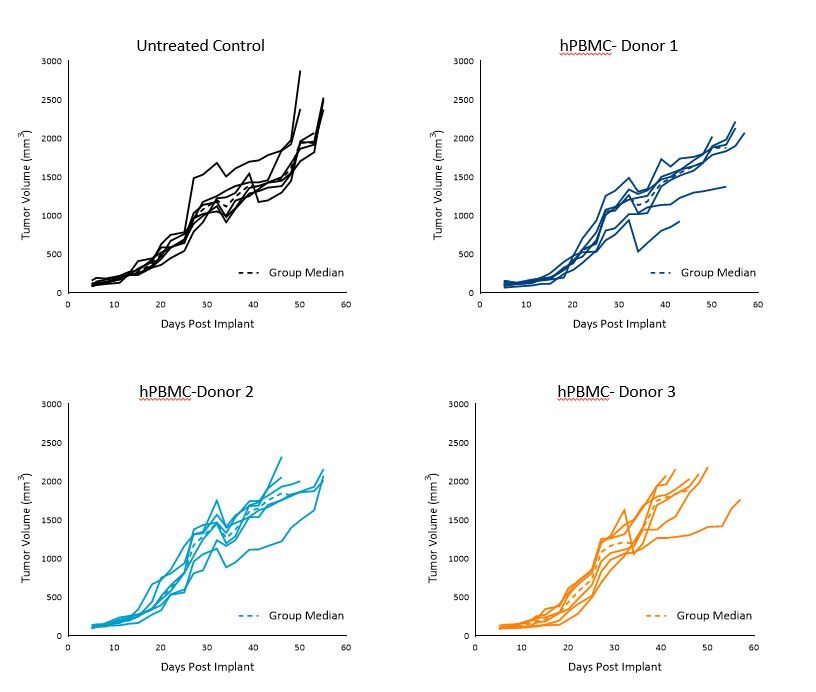
Figure 1. Control growth of MiaPaCa-2 subcutaneous tumors following hPBMC administration
A549 Td in untreated control animals was ~11 days, Td in animals administered hPBMCs ranged from 9 to 12 days across each donor group.
Tumors grew uniformly with hPBMCs from donors 1 and 3 to a mean tumor volume of 1000-1200mm3 by day 40 with some variability in growth seen beyond day 40.
Intragroup variability was most evident in those mice harboring A549 tumors and hPBMCs from donor 2 (Figure 2).
These results suggest that donors 1 and 3 are most suitable for A549 efficacy studies with a TES of 1000mm3.
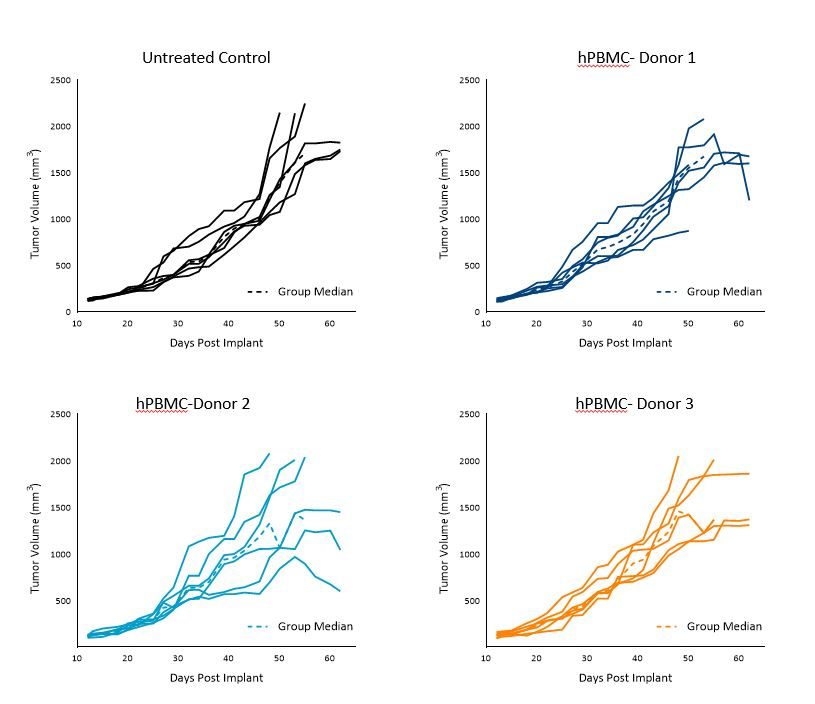
Figure 2. Control growth of subcutaneous A549 tumors following hPBMC administration
Engraftment and Persistence of Human Lymphocytes
Engraftment was assessed on day 28 and either day 38 (MiaPaCa-2) or day 42 (A549) after hPBMC administration by immunophenotypic analysis of human immune cell markers in the peripheral blood from study mice.
Flow cytometry markers included mCD45, hCD45, hCD3, hCD4, and hCD8. Human CD45+ cells, as a percentage of live cells from animals harboring either MiaPaCa-2 (Figure 3A) or A549 tumors (Figure 3B), are presented below and were used as an indicator of hPBMC engraftment.
We found that hCD45+ cells in whole blood were detected across all donors at both timepoints and were in line with published literature.1
While intragroup variability was evident, the average extent of engraftment remained consistent or increased between the first and second timepoint. It did appear that hPBMCs engraftment into animals harboring A549 tumors was somewhat higher than those engrafted into animals harboring MiaPaCa-2 tumors, and this was consistent with our initial model development data.
At this time, it is unknown whether this was a result of normal study-to-study variability or whether there is a model dependency to engraftment.
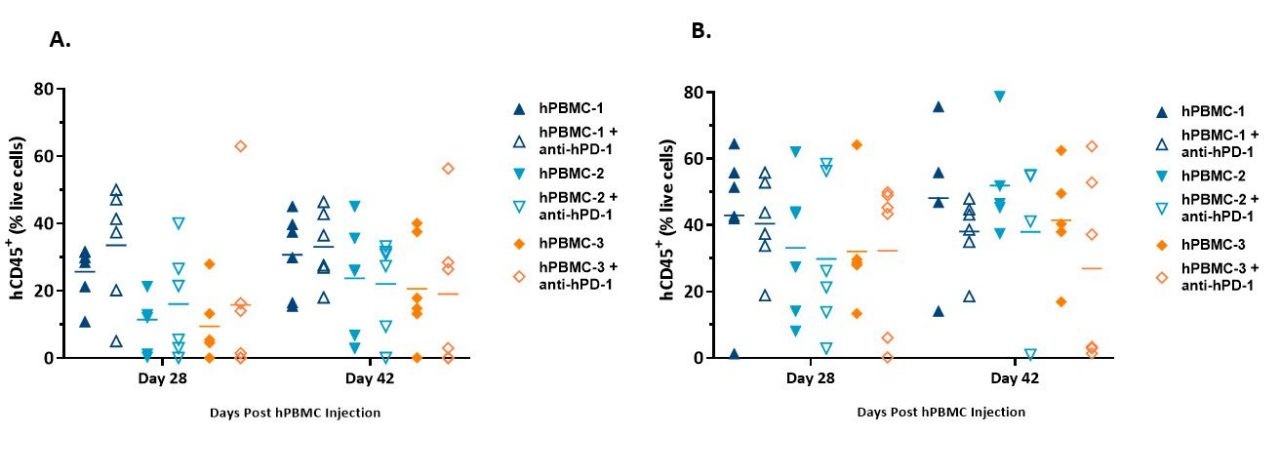
Figure 3. Engraftment of hCD45+ cells detected in whole blood from NSG mice administered hPBMCs and harboring MiaPaCa-2 (A) or A549 (B) xenografts.
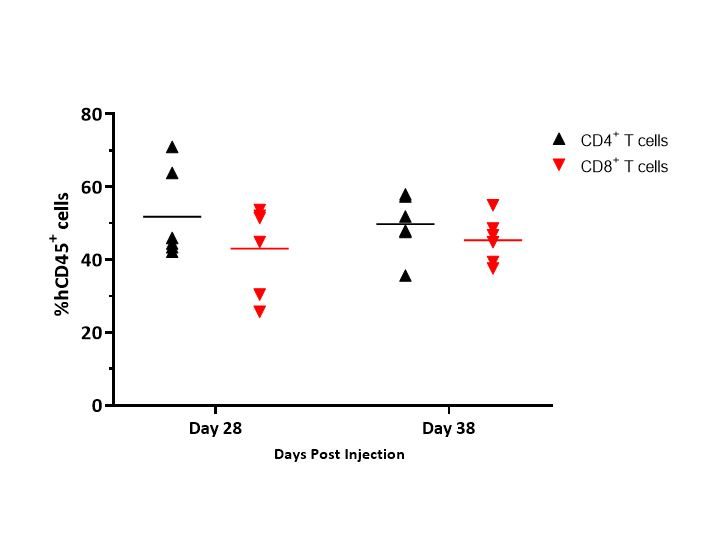
Figure 4 illustrates an example of distribution of CD4+ and CD8+ T cells in whole blood and illustrates that the distribution of T cells was consistent between timepoints.
While these data represent only donor 1 in mice harboring MiaPaCa-2 tumors, similar data was reported across the donors and models tested.
Specifically, distribution of CD4+ and CD8+ T cells ranged from 42-65% and 32-46%, respectively, in MiaPaCa-2-bearing mice, and 51-67% and 22-44%, respectively, in A549-bearing mice (data not shown).
Onset of GvHD symptoms was monitored by body weight and clinical observations; though not confirmed pathologically in these studies, symptoms observed in these models are strongly correlated with the disease2 and with previous data (data not shown).
Response to Pembrolizumab
Interestingly, pembrolizumab treatment in the hPBMC-engrafted mice did not impact MiaPaCa-2 or A549 tumor growth with any of the donors under the conditions tested (Figures 5 and 6).
At the time of this article, there was a paucity of literature regarding in vivo response to pembrolizumab in hPBMC-engrafted humanized mice harboring MiaPaCa-2 tumors.
Notwithstanding, our data is reflective of a wide literature regarding the refractory nature of pancreatic tumors to immuno-oncology agents.3 Response of pembrolizumab against A549 appears moderate as reported previously, and that response seems to be donor dependent.4
Despite the consistent lack of response of pembrolizumab against A549 or MiaPaCa-2, these models can prove useful for therapeutic approaches using rational combination strategies with anti-hPD-1 as there is significant room for improvement and assessment of varying responses across different donors.
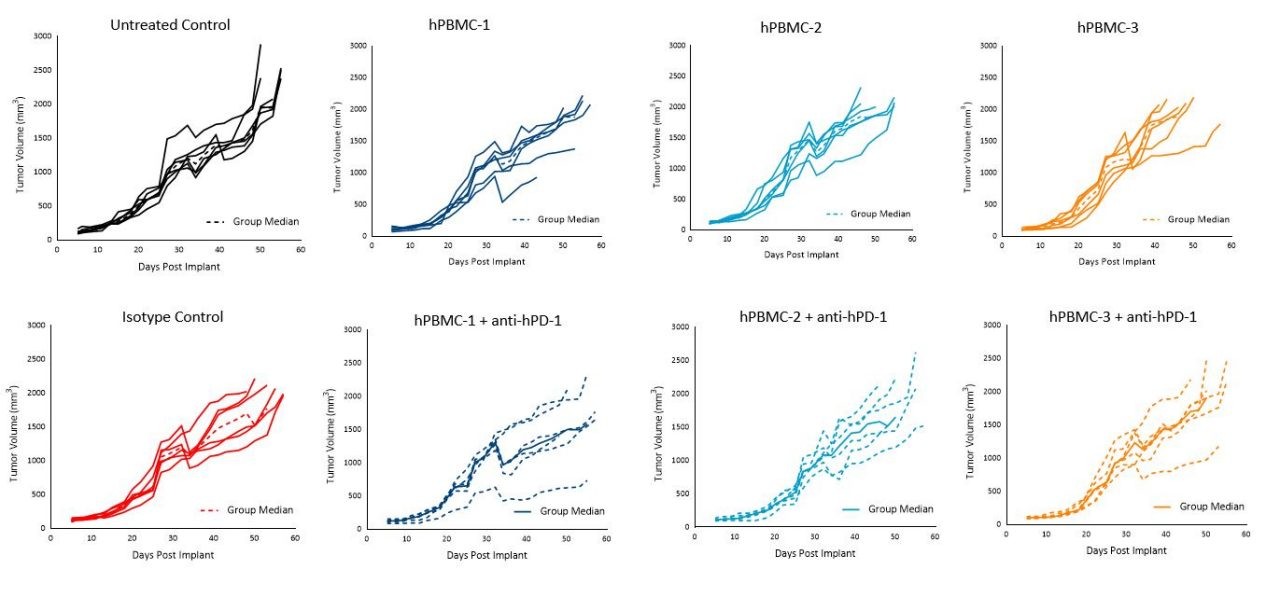
Figure 5. Tumor growth of MiaPaCa-2 subcutaneous tumors following hPBMC administration and treatment with isotype control or pembrolizumab (anti-hPD-1)
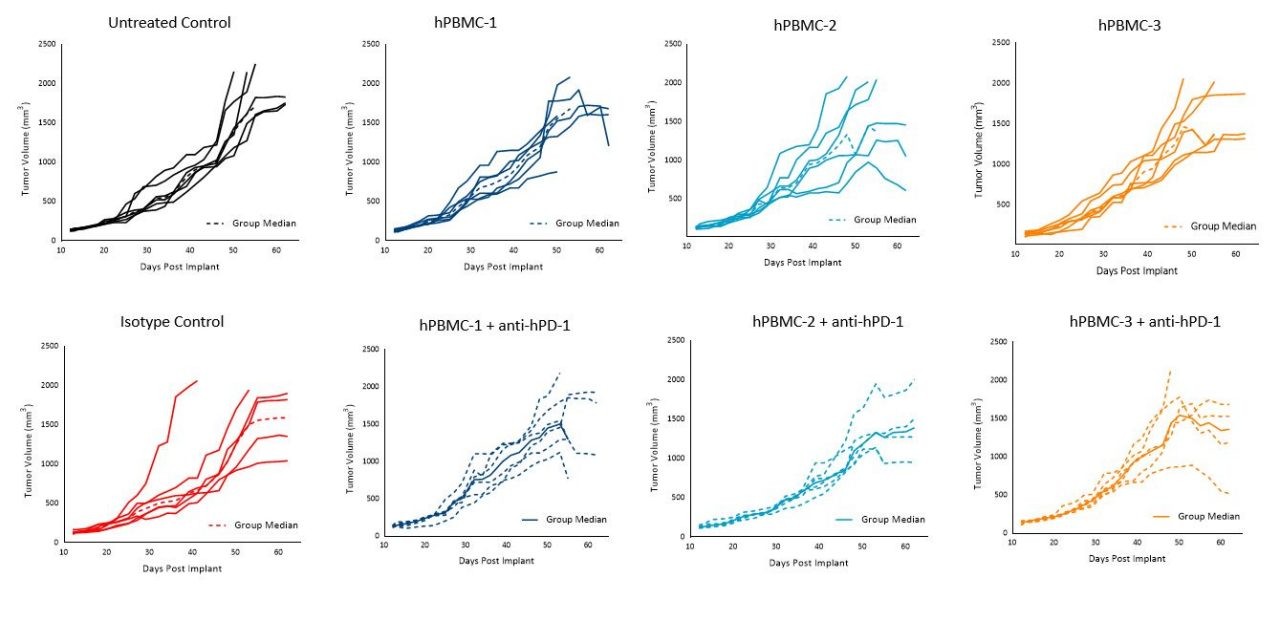
Figure 6. Tumor growth of A549 subcutaneous tumors following hPBMC administration and treatment with isotype control or pembrolizumab (anti-hPD-1)
Administration of hPBMCs to NSG mice results in persistence of human T cells in the mouse with minimal effects on MiaPaCa-2 or A549 tumor growth. This approach represents a powerful preclinical platform to examine the effects of novel human agents that harness human T lymphocytes to direct anti-tumor activity with direct clinical significance.
Future work will demonstrate infiltration of human T cells in the tumor and periphery by flow cytometry with treatment of FDA approved immuno-therapies against human tumor xenografts in NSG mice reconstituted with hPBMCs.
Labcorp has banked supplies of these human PBMC donors to be available for your research needs. Please contact our preclinical oncology scientists to see how hPBMC-engrafted NSG mice can be used for your next translational immuno-oncology study.
References
1.Todd Pearson, Dale L. Greiner, and Leonard D. Shultz. Creation of ?Humanized? Mice to Study Human Immunity. 2008. Curr Protoc Immunol Chapter 15: Unit 15.21
2.Sina Naserian, Mathieu Leclerc, Allan Thiolat, Caroline Pilon, Cindy Le Bret, Yazid Belkacemi, Sébastien Maury, Frédéric Charlotte and José L. Cohen. Simple, Reproducible, and Efficient Clinical Grading System for Murine Models of Acute Graft-versus-Host Disease. 2018. Front. in Immun. (9): 10.
3.Robert J. Torphy, Yuwen Zhu, and Richard D. Schulick. Immunotherapy for pancreatic cancer: Barriers and breakthroughs. 2018. Ann Gastroenterol Surg. 2(4): 274?281.


