Poster
PC-3M-Luc-C6 – a model for prostate carcinoma
Given the poor prognosis (28 percent five-year survival rate) for patients with advanced stage disease (stage III or IV), predictive preclinical models are needed for reliably evaluating treatment options. In most cases of advanced prostate cancer surgical resection is the optimal treatment plan. However, this leaves treating the metastatic lesions with either chemotherapy, radiation, and/or hormone therapy. While subcutaneous xenograft models are valuable, they fail to capture the complexity of the cancer growing in the tissue of origin or the challenges of treating advanced metastatic disease. Orthotopic and metastatic models provide an avenue for not only evaluating the treatment response, but also the impact on the origin tissue and tissue microenvironment, which can be critically important in trying to preserve quality of life.
Orthotopic Modeling
In an effort to address this unmet need, Labcorp has developed an orthotopic and a metastatic prostate model, PC-3M-Luc-C6. This cell line has been transfected with luciferase, which allows for disease and response monitoring through bioluminescence imaging (BLI). The orthotopic model is implanted directly into the prostate gland. Orthotopic tumor growth in the prostate is robust; with established disease present by Day 7 (Figure 1).
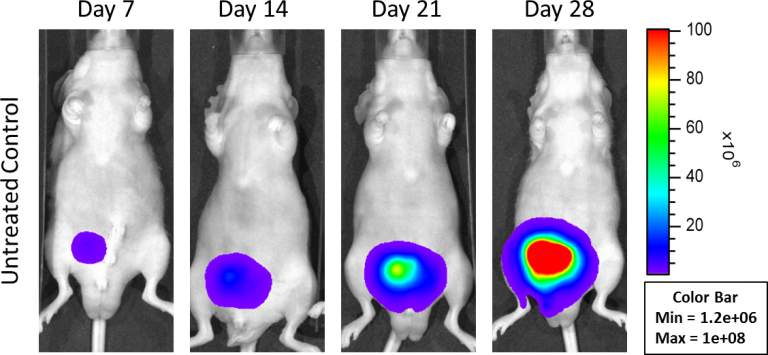
Fig 1: Orthotopic PC-3M-Luc-C6 Human Prostate Carcinoma â Representative Images of Disease Progression â Untreated Control
Treatment with docetaxel was active, producing significant reduction in orthotopic tumor burden (Figure 2). As expected, weekly intravenous treatment with docetaxel (20mg/kg) did induce body weight loss. This lost body weight was recovered following the completion of therapy (Figure 3.)
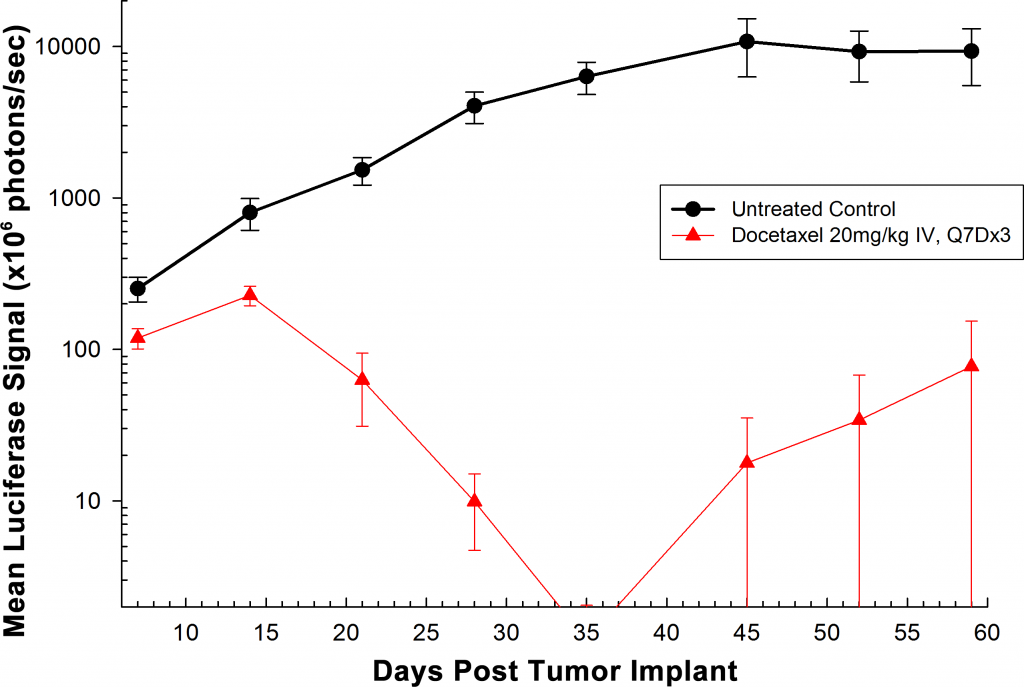
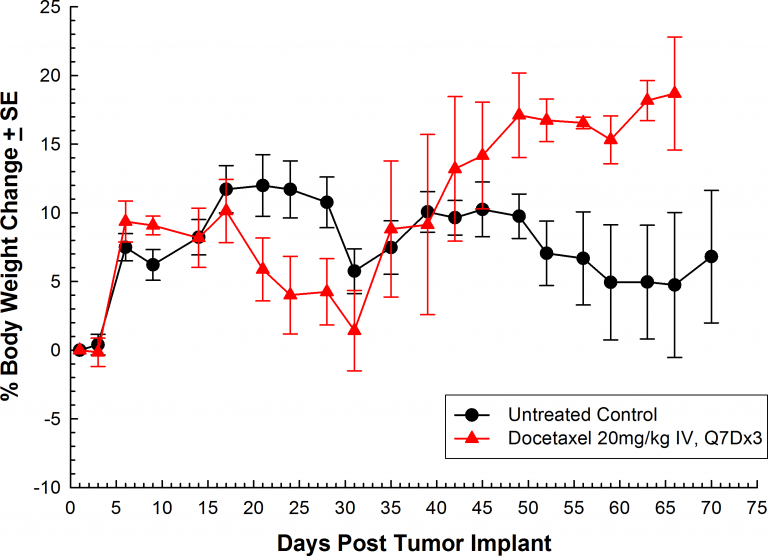
Fig. 2: Orthotopic PC-3M-Luc-C6 Human Prostate Carcinoma â Mean Total Tumor Burden BLI Signal
Fig. 3: Orthotopic PC-3M-Luc-C6 Human Prostate Carcinoma â Mean % Body Weight Change
Metastatic Modeling
In an effort to mimic metastatic disease the PC-3M-Luc-C6 cells are injected into the left ventricle of the heart via an intra-cardiac injection; which distributes cells throughout the entire body and allows them to hone and establish in the bones specifically. Animals are staged and placed into treatment groups based on total bone BLI signal. Progressive disease is monitored with BLI throughout the life of the study (Figure 4).
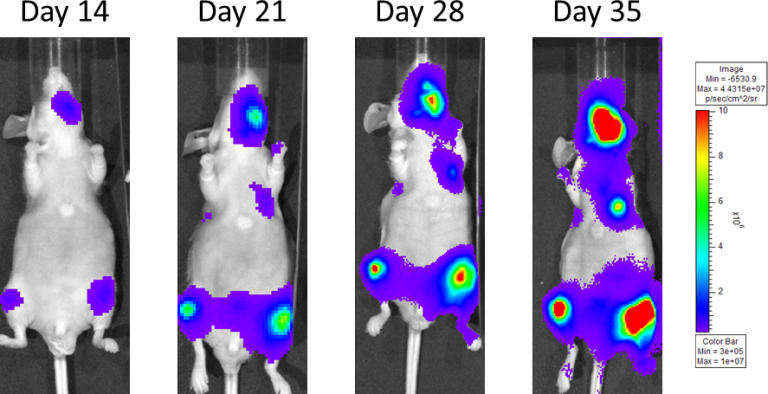
Figure 4: Metastatic PC-3M-Luc-C6 Human Prostate Carcinoma â Representative Images of Disease Progression â Vehicle Control
Weekly treatment with docetaxel (intravenously @ 20mg/kg) was also effective against metastatic disease (Figure 5). Weight loss is common with this model as disease progresses and treatment with docetaxel is known to produce body weight loss during treatment (Figure 6).  However, following the completion of therapy animals in the docetaxel treated group recovered lost body weight. This is yet another indicator that docetaxel treatment was effective.
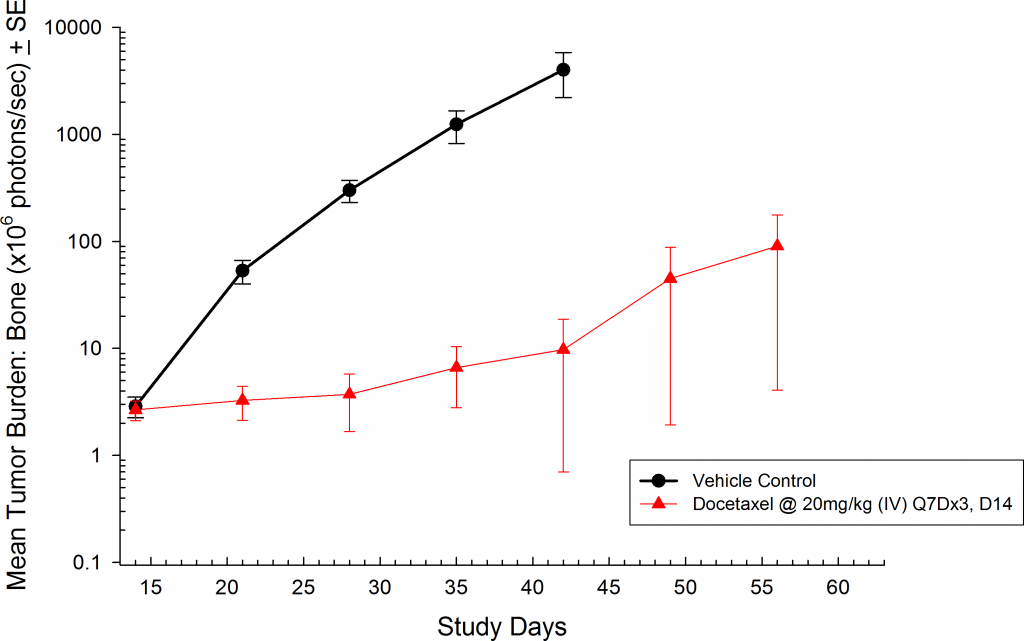
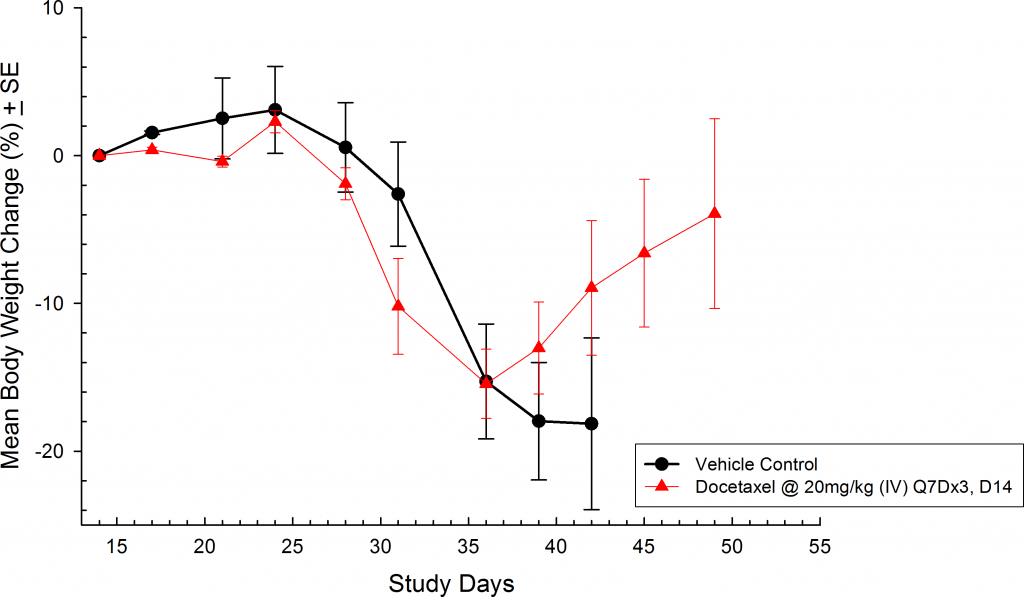
Fig 5: Metastatic PC-3M-Luc-C6 Human Prostate Carcinoma â Mean Total Bone Tumor Burden BLI Signal
Fig 6: Metastatic PC-3M-Luc-C6 Human Prostate Carcinoma â Mean % Body Weight Change
Labcorp has further optimized a long bone specific metastatic model with PC-3M-Luc-C6; information not present here. Please contact us if you are interested in discussing this model or any of our PC-3M-Luc-C6 data further.


