In our November 2017 model spotlight, we described the development of a novel syngeneic mouse model of orthotopic ovarian cancer, ID8-Luc. Ovarian cancer remains a significant unmet medical need with a relative 5-year survival rate of 17% in patients diagnosed with highly advanced stage IV ovarian cancer. While often initially responsive to platinum- and taxane-based chemotherapy, relapse is common in ovarian cancer.
We previously reported a median survival of 35-40 days in the orthotopic ID8 model accompanied by the development of peritoneal ascites. Further investigation of the disease progression in solid tissues using ex vivo bioluminescence imaging (BLI) in animals with advanced disease detected the highest disease burden to localize in the pancreas, spleen, ovaries, and uterine horns.
Checkpoint Inhibitor Testing Using anti-mPD-1 Ab, anti-mPD-L1 Ab, and anti-mCTLA-4 Ab
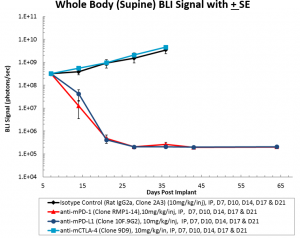
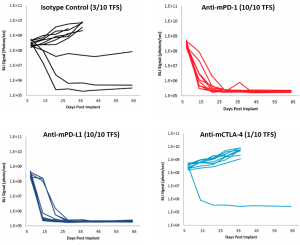
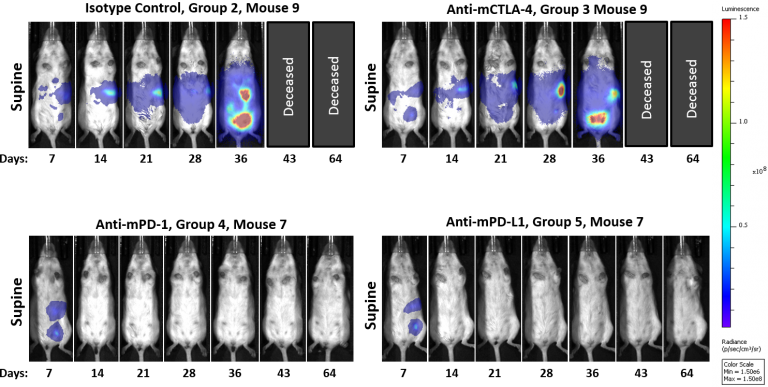
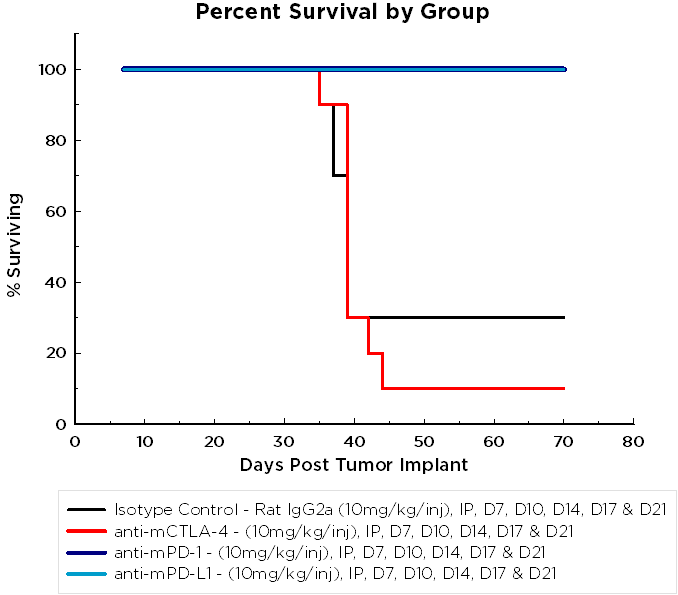
Syngeneic mouse models are of significant value in immuno-oncology research because the mice have an intact immune system that enables the therapeutic manipulation of the immune system to elicit anti-tumor responses. Based on their clinical benefit in human melanoma and non-small cell lung cancer, T cell checkpoint inhibitors, including anti-PD-1, are under active investigation in human ovarian cancer clinical trials. To determine if the ID8-Luc ovarian cancer model was responsive to checkpoint inhibition, we tested anti-mPD-1 Ab, anti-mPD-L1 Ab, and anti-mCTLA-4 Ab in mice with established ID8 peritoneal tumor burden; using BLI to monitor therapeutic response (Figure 1A). Robust tumor regression was observed in the mice treated with both anti-mPD-1 (100% TFS) or anti-mPD-L1 (100% TFS) (Figure 1B). In stark contrast, anti-mCTLA-4 showed no significant activity above that of a rat isotype control antibody. Representative BLI images show extensive disease burden in control and anti-mCTLA-4 treated mice while anti-mPD-1 and anti-mPD-L1 have no detectable disease (Figure 2). The lack of measurable disease was also confirmed by gross necropsy at the end of the study.