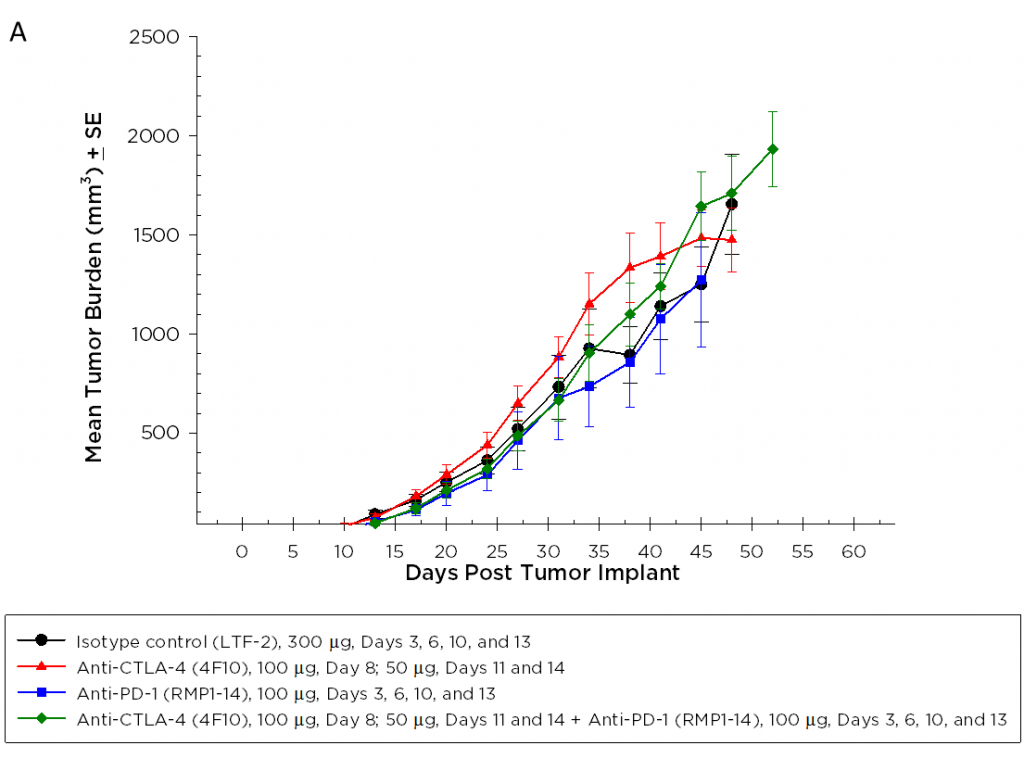
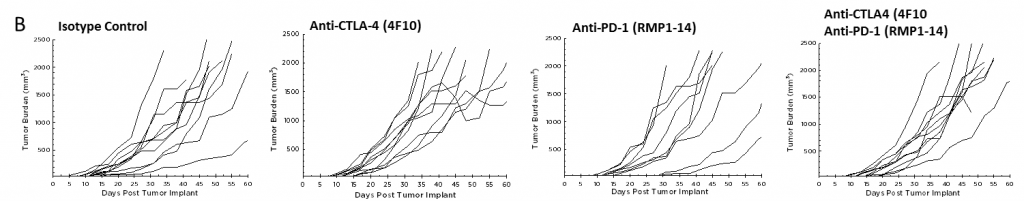
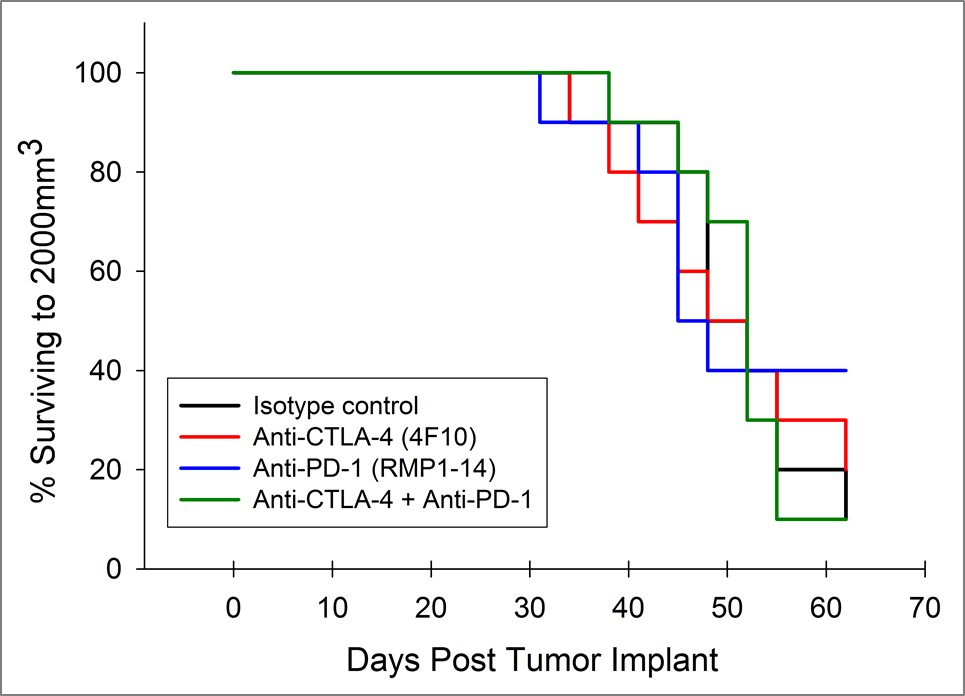
Pancreatic ductal adenocarcinoma (PDAC) is the most prevalent form of pancreatic cancer representing about 95% of all cases. In 2017, approximately 50,987 people will be diagnosed with PDAC in the United States, and approximately 40,936 patient deaths will occur, making PDAC one of the most lethal forms of cancer. Combination anti-metabolite and anti-mitotic taxane based chemotherapy is the standard of care in the US, but these treatment options historically only improve overall survival by weeks. There is a critical unmet medical need for novel therapeutic approaches to treat pancreatic cancer.
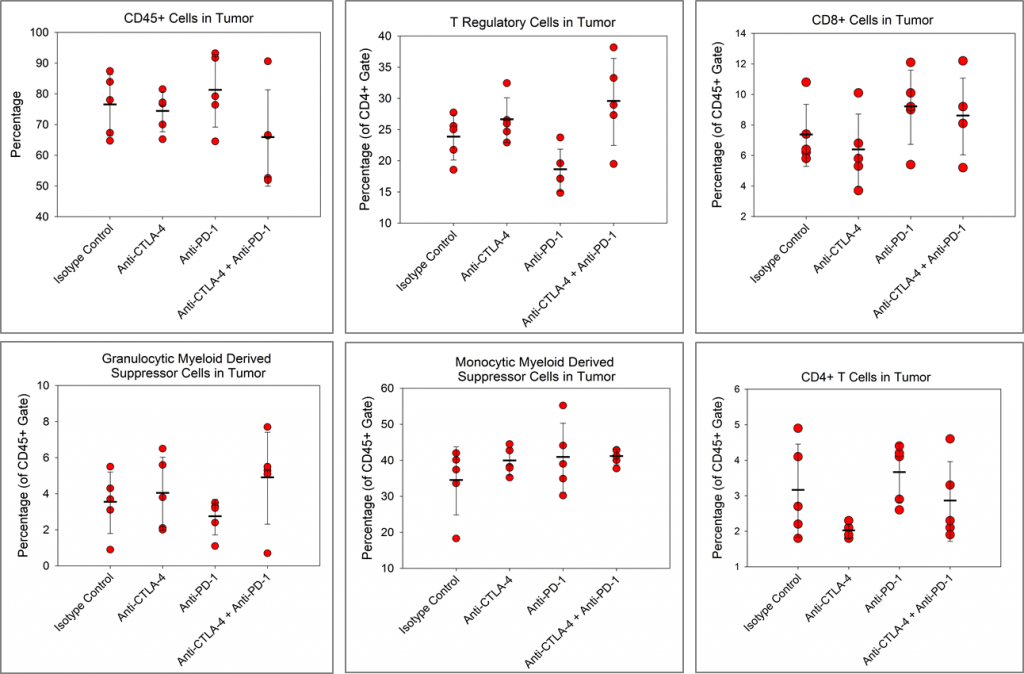
Because so many novel approaches to treat pancreatic cancer have met with disappointing clinical outcomes, many drug discovery scientists have turned to immunotherapy in pancreatic cancer based on the recent successes of harnessing the immune system to treat other cancers. Although there are a limited number of syngeneic mouse pancreatic cancer cell lines, Labcorp has characterized the Pan02 PDAC model for immuno-oncology applications. Pan02 was derived from C57BL/6 mice given orthotopic 3-methyl-cholanthrene and is refractory to many standard chemotherapeutic agents.1 Pan02 contains a loss of function mutation in the SMAD4 gene that is functionally similar to inactivating mutations in approximately 30% of human pancreatic cancers.2
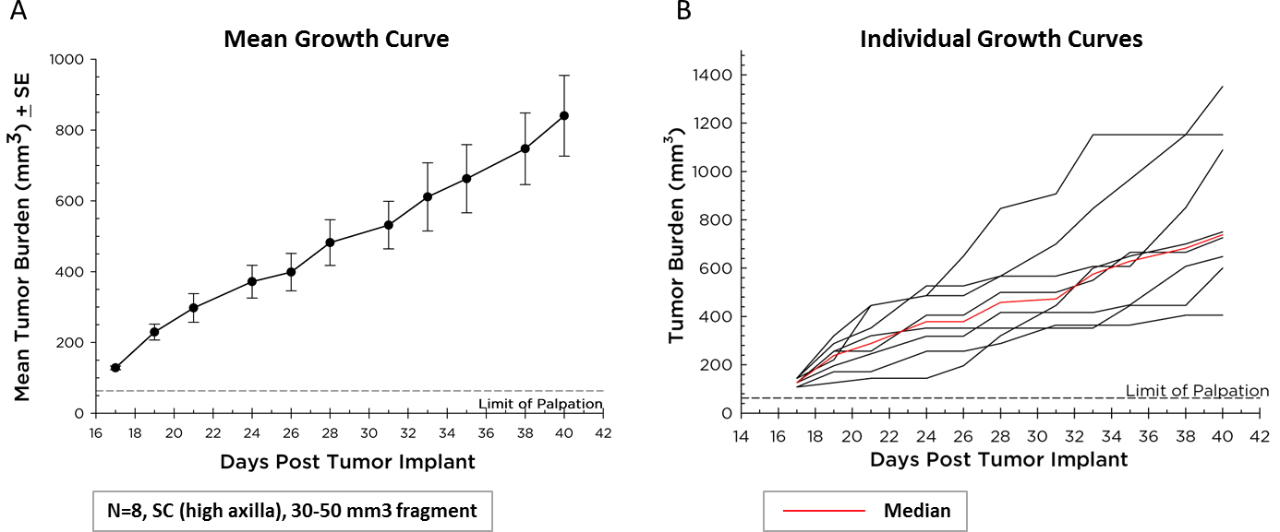
Mean and Individual Growth Curves
Labcorp maintains Pan02 as a transplantable fragment model, and its mean growth kinetics (Figure 1A) and individual animal growth curves (Figure 1B) are illustrated. Pan02 has a mean tumor doubling time of six days which is slower than most syngeneic mouse cell line tumors. This slower growth may make it more tractable for immunotherapy since there is time for therapeutics to modify the immune system and elicit anti-tumor activity prior to tumors reaching euthanasia criteria.