Reduce your administrative burden, protect your supply chain and free resources for innovation with our one-stop shop. Find out more about our antibody critical reagent service by listening to our latest webinar.
-
Single, global solution for antibody reagent portfolio management
-
Delivery of consistent, high-quality, reproducible reagents across the development life cycle
-
Experienced team proactively manages portfolio to reduce administrative burden and de-risk your program
Antibody critical reagents are essential for regulated (GxP) bioanalytical assays pivotal to the development and manufacturing of biotherapeutics. Bioanalytical assay performance depends on reagents that are high-quality, well characterized, consistent between lots and available when needed. If your antibody critical reagents don’t meet these standards, your assays may fail and your development schedules can be derailed. This can potentially cost you time, money and reputation.
Introducing a unique, end-to-end solution that de-risks your antibody critical reagent supply chain
- Integrated, one-stop shop for generation, characterization, storage and distribution of your antibody critical reagents
- High-quality, reliable and sustainable reagents delivering bioanalytic assay success throughout your biotherapeutic’s development life cycle
- Experienced team to proactively manage your inventory so that you have in-date antibody critical reagents when and where you need them
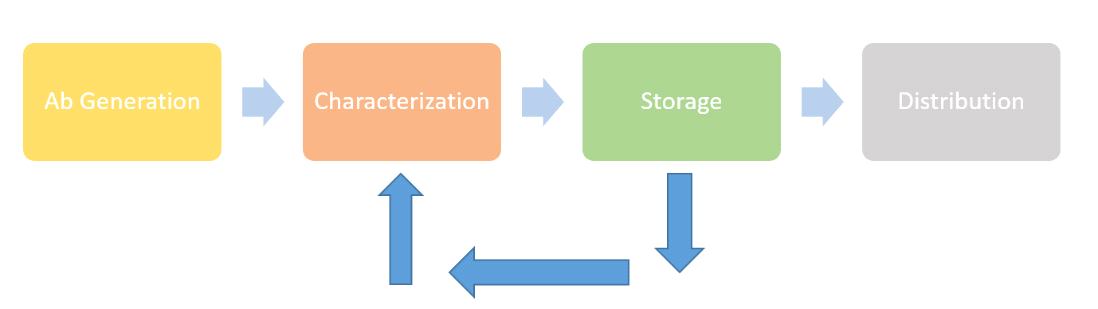
Quality and reliability are built into all stages of our reagent portfolio management solution
We’ve built each aspect in our process to work as a stand-alone offering. That means we’ve optimized each stage to deliver the very best service and facilities. So, whether you need innovations in generation, our comprehensive suite of bioanalytic methods, access to our world-class storage facilities and capacity or proactive inventory management, you’ll get the very best from us. In addition, we have built-in clear risk mitigation strategies at each step to guarantee that you always have a sufficient supply of the highest-quality antibody critical reagents.
Generation
- Polyclonal antibodies
- Monoclonal antibodies
- (sub-bullet – hybridoma)
- (sub-bullet – B-cell cloning)
- Antibody purification and labeling
Characterization
- Comprehensive toolbox of analytical methods
- Quality management system ensures consistent reagents
- Certificate of analysis, established SOPs for re-characterization
Storage
- Continuous automated monitoring system
- Freezer validation and management
- Secured facilities
- Monitored, controlled access areas
- F5 tornado rated facility
- Business continuity plans