01 Mar 2018
Author: Erin Trachet | Sr. Scientific Advisor, Oncology / Sr. Manager, Proposal Development
Date: March 2018
As we presented in last month’s model spotlight, lung cancer is a devastating disease and is the leading cause of cancer death in the US and worldwide.1 The research community continues to look for new models that will aid in lung cancer research. The ATCC (a widely used cell repository) currently has over 100 different human derived lung cancer cell lines.
Because lung cancer is so prevalent, there are numerous opportunities for new human lines to be acquired and characterized. Scientists have become highly skilled in distinguishing the mutations that drive increased proliferation from those that suppress tumor growth. This information is key to developing novel therapies to treat lung cancer.
Labcorp continues to investigate new and/or unconventional cell lines and understand their importance to our client’s research. In this Model Spotlight we will discuss a few of these lines and how we position our expertise to be a valuable resource to our clients as they look to develop future treatments.
NCI-H3122
NCI-H3122 was derived in 1981 from a primary bronchioalveolar carcinoma of the lung from a 52-year-old male taken prior to treatment. This cell line harbors the EML4-ALK fusion protein (echinoderm microtubule-associated protein-like 4 fused with anaplastic lymphoma kinase gene) and has recently been identified in ~7% of Japanese NSCLC (non-small cell lung cancer) patients and ~4.5% of all NSCLC cases. The ALK gene is a target of interest given its ability to be oncogenic. As NCI-H3122 contains EML4-ALK and is highly sensitive to single agent ALK inhibitors it appears that its survival is mediated by a more ALK-addicted pathway.2 As a preclinical model this cell line would be suitable for screening novel ALK inhibitors that are not dependent on EGFR, or other pathways involved in NSCLC. The model has been developed as a subcutaneous (SC) implant; however, we have also implanted this line intracranially to mimic the clinical setting where advanced NSCLC metastasizes to the brain. Subcutaneous tumor growth is reliable with minimal animal-to-animal variability. The tumor volume doubling time in the SC setting is ~7 days and mice typically reach an evaluation size of ~750mm3 in about 25 days. Initial data with NCI-H3122 implanted intracranially is encouraging, in a pilot study we have seen 100% take rate and a tumor volume doubling time, based on volumetric assessment, of ~7 days, which is consistent with the SC growth rate (See Figures 1 and 2). As with many intracranial models, morbidity (body weight loss and overall clinical observation) is used to define the overall survival. For this cell line the median day of death is ~35 days (See Figures 2, 3, and 4).
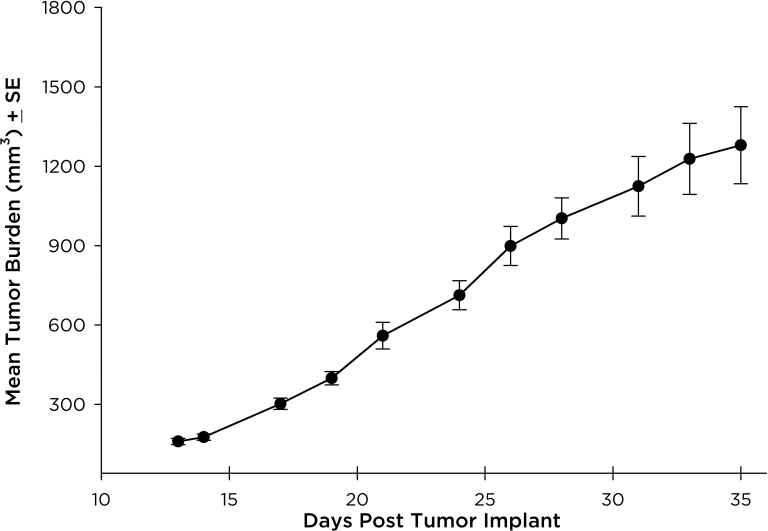
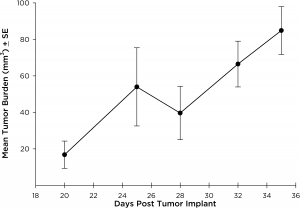
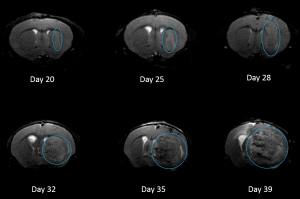
Fig. 1: Subcutaneous NCI-H3122 Mean Tumor Burden
Fig. 2:Intracranial NCI-H3122 Mean Tumor Growth By MRI
Fig. 3: % Body Weight Change Following Intracranial Implant of NCI-H3122
Fig. 4: H3122-3160 Representative Image
NCI-H1703
NCI-H1703 was derived from a stage 1 lung squamous cell carcinoma of a 54-year-old Caucasian male smoker. This cell line is of interest to the research community based on the high levels of platelet-derived growth factor receptor α (PDGFRα) amplification. NCI-H1703 is one of very few NSCLC models that have this expression profile and are sensitive to sunitinib in vitro. Clinically, lung cancer patients that express PDGFRα are associated with more aggressive tumor biology and worse prognosis.3 Therefore, this model is suitable for evaluating novel angiogenic targets such as VEGF as well as novel PDGFRα targets independent of the cell surface proliferation pathways. The model is most commonly utilized following subcutaneous (SC) implant, however, we have also transfected this line with luciferase to allow for bioluminescence imaging to monitor disease progression following direct implant into the lung. Tumor growth is reliable following either SC or orthotopic (OT) lung implant. In both implant sites there is minimal animal-to-animal variability, tumor volume doubling is every 6 days (SC) and 11 days (OT). While the tumor growth rates are different for each implant site the mice typically reach evaluation size (~750mm3 or 1.0E+09 p/s) in approximately 30 days post implant (See Figures 5 [SC], 6, 7, and 8 [OT]).
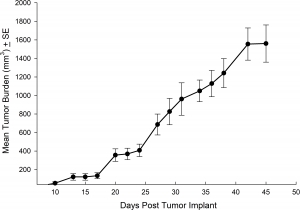
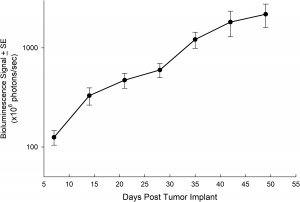
Fig. 5: Subcutaneous NCI-1703 Mean Tumor Burden
Fig. 6: Mean Tumor Burden Following Orthotopically Implanted NCI-1703
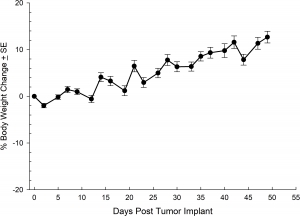
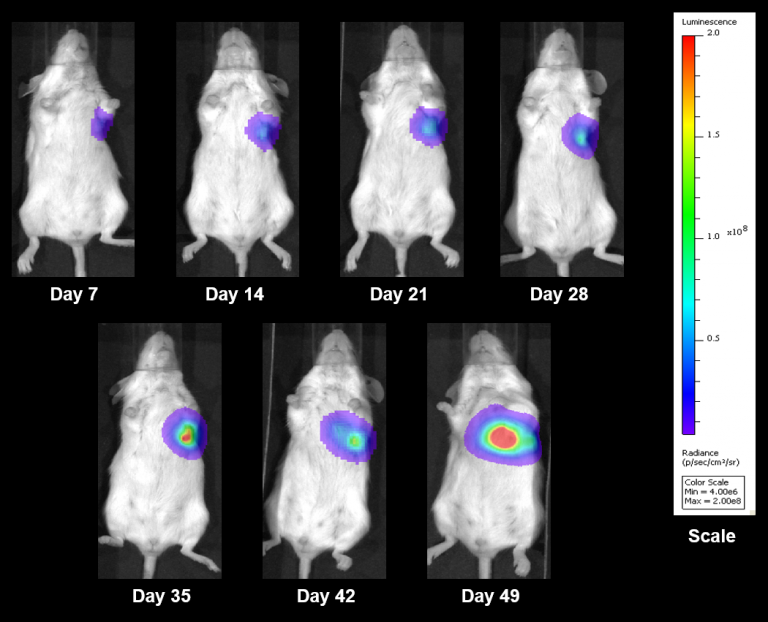
Fig. 7: % Body Weight Change Following Orthotopically Implanted NCI-1703
Fig. 8: Representative BLI Images Following Orthotopically Implanted NCI-1703
PC-9
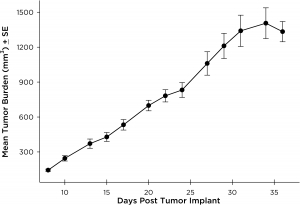
PC-9 was isolated from a male lung adenocarcinoma patient in 1989. PC-9 has been reported to be very sensitive to gefitinib and other EGFR tyrosine kinase inhibitors. However, it has been shown that prolonged exposure of PC-9 cells to EGFR inhibitors can result in acquisition of the T790M mutation and a resistant cell line. This model would be valuable when evaluating next generation EGFR inhibitors or possibly in the creation of a resistant version to evaluate alternative treatment approaches. We have developed PC-9 as a subcutaneous model that demonstrates reliable growth with minimal animal-to-animal variability. The tumor volume doubling time is ~7 days and the tumors typically reach evaluation size (~750mm3) in approximately 28 days post implant (See Figures 9).
Please contact us if you are interested in discussing any of our human NSCLC models.
Investigate our full list of human xenograft models, including our extensive NSCLC Bank.
Fig. 9: Subcutaneous PC-9 Mean Tumor Burden
