01 Feb 2023
Date: February 2023
Adoptive cell therapy (ACT) with T-cells reprogrammed to express chimeric antigen receptors (CAR T-cells) bind specific antigens on the surface of cancer cells and trigger a cytotoxic response. CAR T-cell therapies have been successful in patients with hematological malignancies; however, patients remain at risk of relapse due to short-term persistence or non-expansion of CAR T-cells.1,2 This requirement for persistence is more evident in solid tumors as the hostile tumor microenvironment induces T-cell exhaustion.3,4 Studies have found that genomic and phenotypic features of CAR T-cells were major determinants of their persistence. Clinical results have shown that stemness and a memory-cell-like phenotype can promote sustained in vivo persistence of adoptively transferred CAR T-cells.3,4
For example, CAR T-cells expressing a CD28 costimulatory domain exhibited an increased expression of T-cell exhaustion-related genes and were reported to only persist up to three months in clinical trials of patients with relapsed or refractory acute lymphoblastic leukemia (R/R ALL).5 Conversely, CAR T-cells containing the 4-1BB costimulatory domain with the same antigen specificity reduced the exhausted phenotype and were found to persist for up to five years and more than six months in almost all evaluated patients with R/R ALL.5 Preclinical studies investigating 4-1BB expressing CAR constructs have shown long CAR T-cell persistence and a higher proportion of memory T-cells.6
Therefore, establishing reliable methods of tracking not only CAR T-cell numbers but also activation/exhaustion markers such as costimulatory molecules and inhibitory receptors on the CAR T-cell surface is essential to determine long-term efficacy and T-cell fitness over time after infusion into the host. Following up on our basic PersistenceT™ panel (Spotlight: Determining preclinical CAR T-cell persistence by flow cytometry), we have now developed customizable Expanded PersistenceT panels to perform a deeper longitudinal analysis of both circulating CAR T-cells count and activation/exhaustion marker expression.
Table 1. Expanded PersistenceT panel of antibodies and descriptions of their use
PersistenceT Panel Antibodies
Antibody/Beads | Description |
Standard Components | |
mCD45 | Pan-hematopoietic cell marker (mouse) |
hCD45 | Pan-hematopoietic cell marker |
hCD3 | Pan-T-cell marker |
hCD4 | CD4+ T-cell marker |
hCD8 | CD8+ T-cell marker |
Counting Beads | Provides absolute counts |
Modular Additions (Available Upon Request) | |
CAR Probe | CAR T marker |
Viability | Live cell marker |
Expanded PersistenceT Panel 1 Antibodies*
Antibody | Description |
hCD25 | Activation marker |
h4-1BB | Activation marker |
hICOS | Activation marker |
hTIM-3 | Checkpoint marker |
hPD-1 | Checkpoint marker |
hLAG-3 | Checkpoint marker |
Expanded PersistenceT Panel 2 Antibodies*
Antibody | Description |
hCD27 | Activation marker |
hCD69 | Activation marker |
hICOS | Activation marker |
hTIM-3 | Checkpoint marker |
hPD-1 | Checkpoint marker |
hLAG-3 | Checkpoint marker |
* List is not exhaustive, ask about other markers
We evaluated the activity and persistence of CD19-targeted CAR T-cells in the disseminated NALM6-Luc ALL model using flow cytometry with the Expanded PersistenceT panel 1. Female NSG mice (n = 8 per group) were implanted with 5.00+05 NALM6-Luc-mCh-Puro cells/implant and were treated with 1E+07 CD19 CAR T-cells or untransduced (UTD) T-cells. Human T-cells in the peripheral blood were then quantified and analyzed over the study duration by flow cytometry using the Expanded PersistenceT panel.
Tumor growth in this NALM6-Luc ALL model is delayed by CD19 CAR T-cell treatment, correlating with increased host survival times (Figure 1).
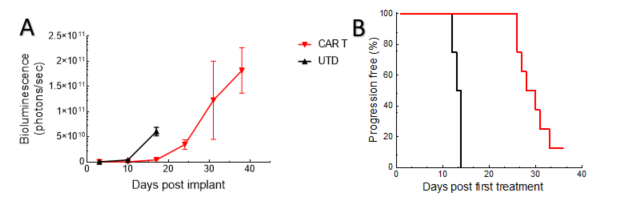
Figure 1. Tumor burden (whole body bioluminescence imaging signal) (A) and time to progression (B) for animals following adoptive transfer using UTD and CAR T-cells.
CAR T-cell numbers in the NALM6-Luc tumor-bearing mice contract in the blood and then return in the late phase of the study when treatment no longer controls tumor growth (Figure 2).
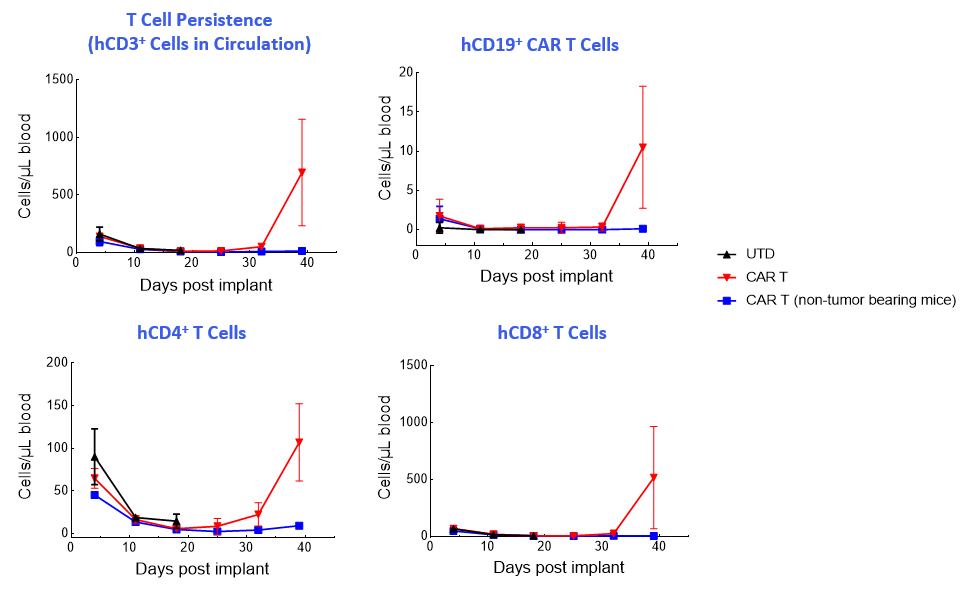
Figure 2. T-cell enumeration over study duration.
The Expanded panel analysis revealed that uncontrolled tumor growth coincided with reduced T-cell activation marker expression (CD25 and ICOS) and increased exhaustion marker expression (PD-1 and LAG-3) (Figure 3).
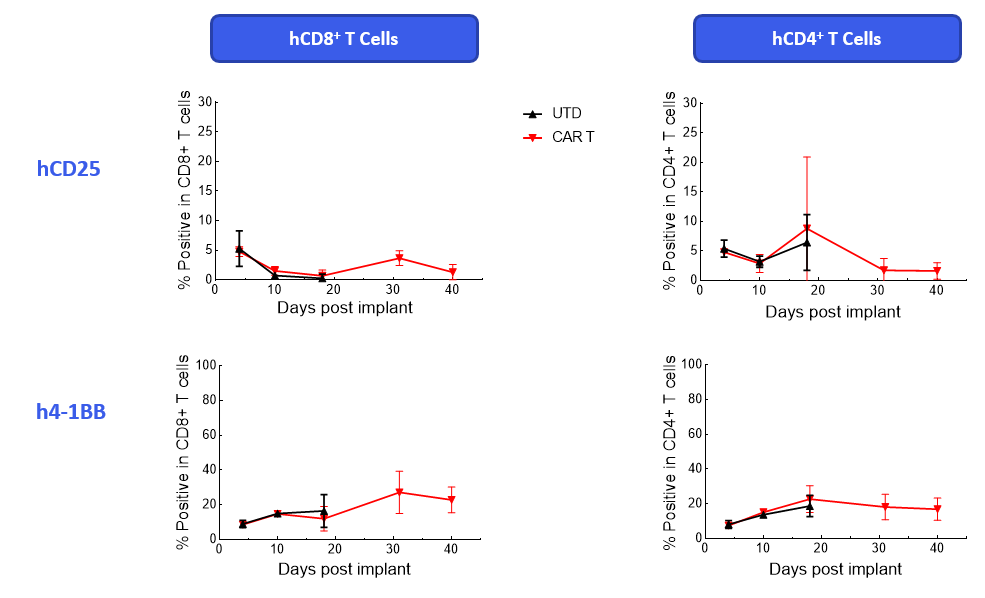
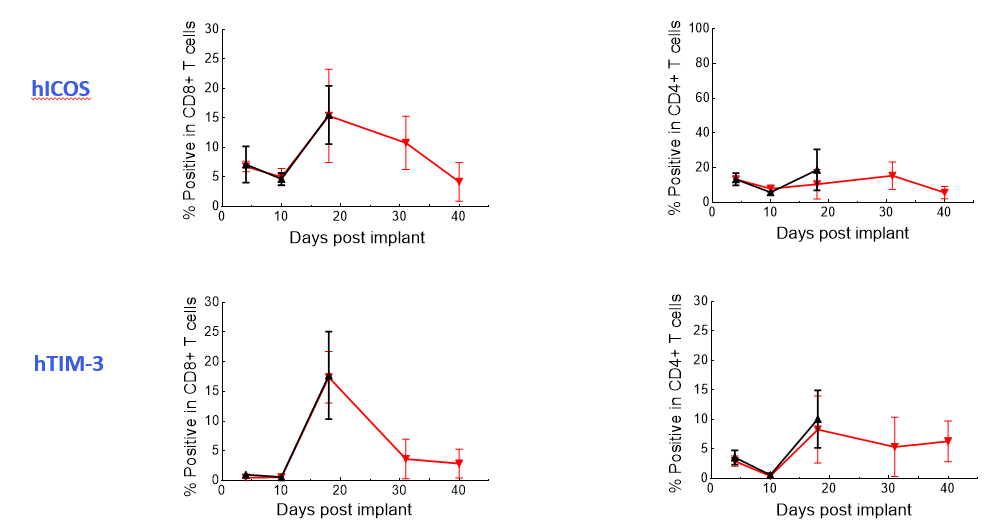
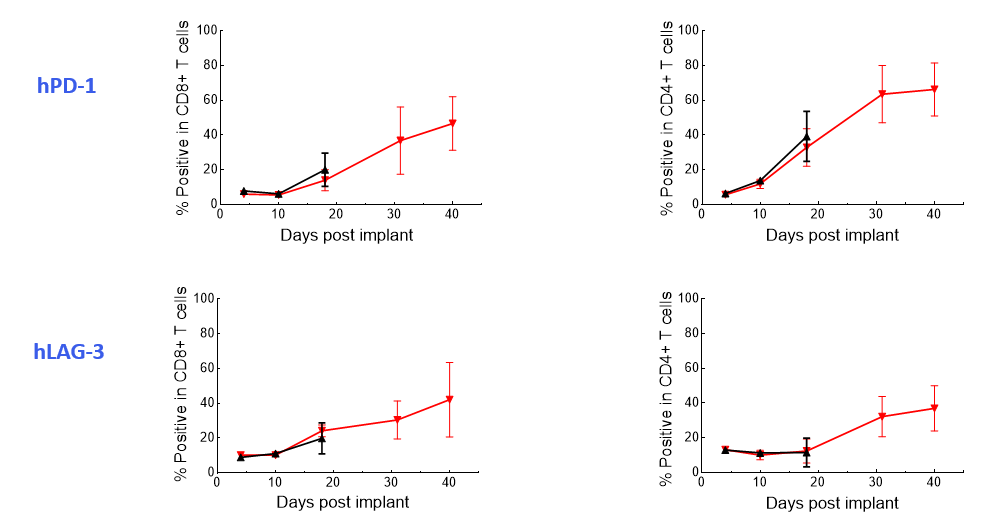
Figure 3. Longitudinal analysis of activation/exhaustion markers expression in NALM6-Luc tumor-bearing mice treated with CAR T and UTD cells using the Expanded PersistenceT panel 1.
The data suggest that the treatment-induced effects observed were independent of graft versus host disease because the CAR T-cell phenotypes were not recapitulated in non-tumor-bearing mice (Figure 4).
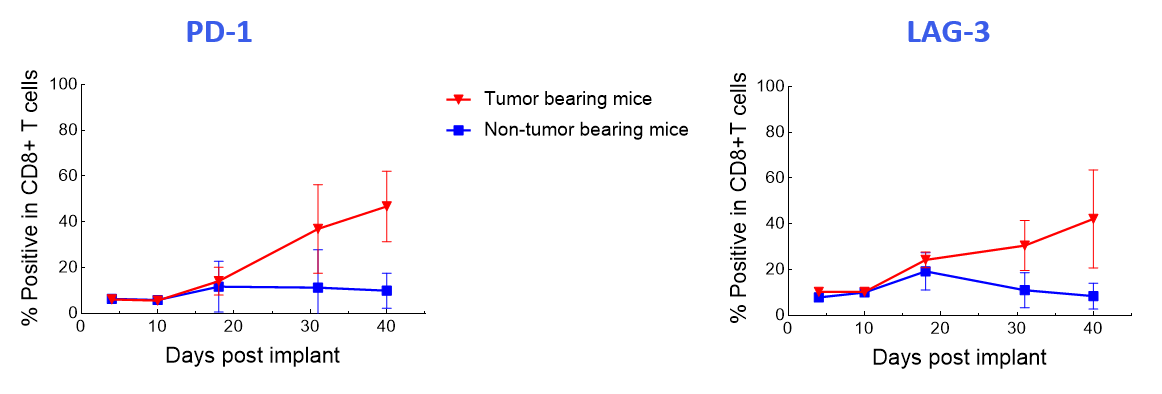
Figure 4. Longitudinal analysis comparing PD-1 and LAG-3 expression in human CD8+ T-cells of CAR T-cell-treated tumor-bearing and non-tumor-bearing mice using the Expanded PersistenceT panel 1.
CAR T-cells continue to evolve through modifications of the CAR to include factors that enhance T-cell expansion, antitumor activity and persistence.7 In vivo CAR T persistence in preclinical studies is a surrogate marker of long-term clinical efficacy of CAR T-cell therapy. The Expanded PersistenceT panel, therefore, offers an excellent tool to determine the clinical success of your CAR T-cell therapy.
Labcorp’s Preclinical Oncology department has the experience to perform your CAR cell therapy and other ACT studies using any commercially available mouse strains including NSG mice; and under certain conditions, we can accept specialty mice as well. To begin a discussion about your next project, contact the scientists on Labcorp’s preclinical oncology team.
