01 May 2018
Author: Sheri Barnes, PhD | Director, Scientific Development
Date: May 2018
Breast cancer is the most common cancer among women in the United States and is the fourth leading cause of cancer death. In 2017, an estimated 252,710 new cases were diagnosed (15% of all new cancers) and 40,610 patient deaths occurred. Early detection initiatives together with improved treatment options have resulted in increases in the 5-year overall survival rate from 75% in 1975 to over 90% today.[1] Despite the favorable survival statistics, metastatic disease continues to be a treatment challenge and often results in death. For this reason, the continual development of new treatments for breast cancer is necessary.
Advancements in Using Immuno-oncology Combination Therapies for Breast Cancer
While several treatment options are available to fight hormone- or Her2-driven breast cancers, options are somewhat limited for triple negative breast cancer patients who cannot take advantage of these targeted therapies. Adding to this problem, despite success with immunotherapies in treating melanoma and lung cancer, breast cancer has proven to be especially difficult to treat by checkpoint blockade or other immunotherapies. However, with the focused effort in recent years towards immuno-oncology approaches to breast cancer, emerging clinical data is showing promise, particularly in combination therapy.[2] To help drive this research forward, we have developed the EMT-6 syngeneic breast tumor model. Derived from a transplanted hyperplastic alveolar nodule in BALB/c mice,[3] this model takes advantage of the complete mouse immune system and serves as a powerful tool in the immuno-oncology space.
Testing Combination Therapies Using the EMT-6 Model
Breast cancer is the most common cancer among women in the United States and is the fourth leading cause of cancer death. In 2017, an estimated 252,710 new cases were diagnosed (15% of all new cancers) and 40,610 patient deaths occurred. Early detection initiatives together with improved treatment options have resulted in increases in the 5-year overall survival rate from 75% in 1975 to over 90% today.[1] Despite the favorable survival statistics, metastatic disease continues to be a treatment challenge and often results in death. For this reason, the continual development of new treatments for breast cancer is necessary.
Advancements in Using Immuno-oncology Combination Therapies for Breast Cancer
While several treatment options are available to fight hormone- or Her2-driven breast cancers, options are somewhat limited for triple negative breast cancer patients who cannot take advantage of these targeted therapies. Adding to this problem, despite success with immunotherapies in treating melanoma and lung cancer, breast cancer has proven to be especially difficult to treat by checkpoint blockade or other immunotherapies. However, with the focused effort in recent years towards immuno-oncology approaches to breast cancer, emerging clinical data is showing promise, particularly in combination therapy.[2] To help drive this research forward, we have developed the EMT-6 syngeneic breast tumor model. Derived from a transplanted hyperplastic alveolar nodule in BALB/c mice,[3] this model takes advantage of the complete mouse immune system and serves as a powerful tool in the immuno-oncology space.
Testing Combination Therapies Using the EMT-6 Model
In this spotlight, we present data from our initial efficacy study with the EMT-6 model. These data should enable the design of rational combination studies with novel therapeutics while additional studies are performed. Control tumor growth for the EMT-6 model is shown in Figure 1. The median doubling time is 5.5 days on average, allowing for a therapeutic window of at least three weeks with which to elicit anti-tumor activity. The growth rates of untreated tumors and those treated with an isotype control antibody (rat IgG2b) are nearly superimposable. To determine the response of EMT-6 to immunomodulatory therapies, mice bearing EMT-6 tumors were treated with checkpoint blockade (anti-mPD-1 and anti-mPD-L1), and anti-mCD137, a costimulatory molecule. Additionally, as focal radiation is oftentimes used to treat breast cancer, we included modeling of radiation monotherapy and combination therapy.
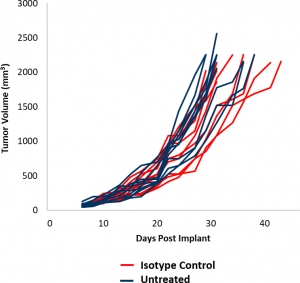
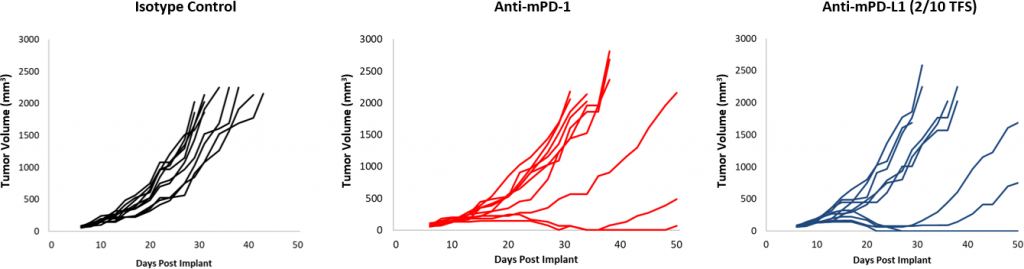
Fig. 2: EMT-6 Response to Checkpoint Inhibitors.
Responses to checkpoint blockade were moderate overall compared to control (Figure 2). Although a subset of animals did respond, treatment with anti-mPD-1 and anti-mPD-L1 still offers room for improvement in combination. Further, a focal radiation dose of 10Gy, which causes significant tumor growth delay in other syngeneic models, offered only modest activity in EMT-6. Addition of anti-mPD-1 to this regimen offers some additive impact, yet still leaves room for improvement in triple combination. In contrast, treatment with anti-mCD137 monotherapy results in a strong response (4/10 TFS) against EMT-6. Combination with focal radiation offered nominal improvement (5/10 TFS) over anti-mCD137 monotherapy (Figure 3). These data demonstrate the advantage of using the EMT-6 model to investigate dual or triple combination strategies which utilize radiation, checkpoint blockade, and/or costimulatory molecule agonists.
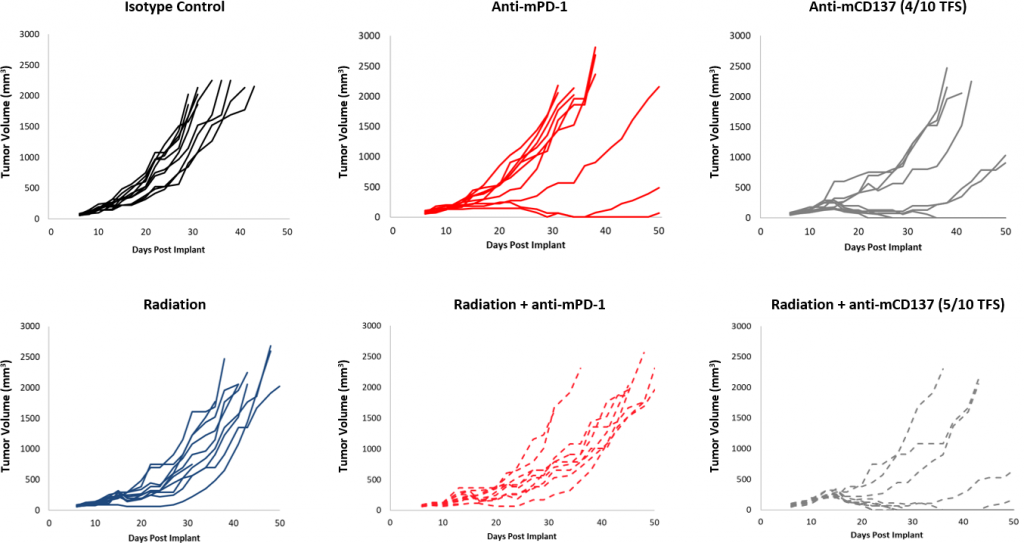
Fig. 3: EMT-6 Response to Focal Radiation Combination Therapy
Composition of Infiltrated Immune Cells Within the Tumor
When interrogating models for immuno-oncology purposes, it’s also important to know the composition of infiltrated immune cells into a tumor. To this end, tumors between 220-385 mm3 were examined for infiltrated T cells and myeloid cells expressed as percentages of total CD45+ cells (Figure 4). The myeloid compartment across all tumors was evenly distributed across M2 macrophages, monocytic MDSCs, and granulocytic MDSCs. M1 macrophage infiltrate was comparatively lower than other myeloid derived cells. This composition is suggestive of an immunosuppressive microenvironment which could help explain the limited response with most of the immunotherapy regimens tested.
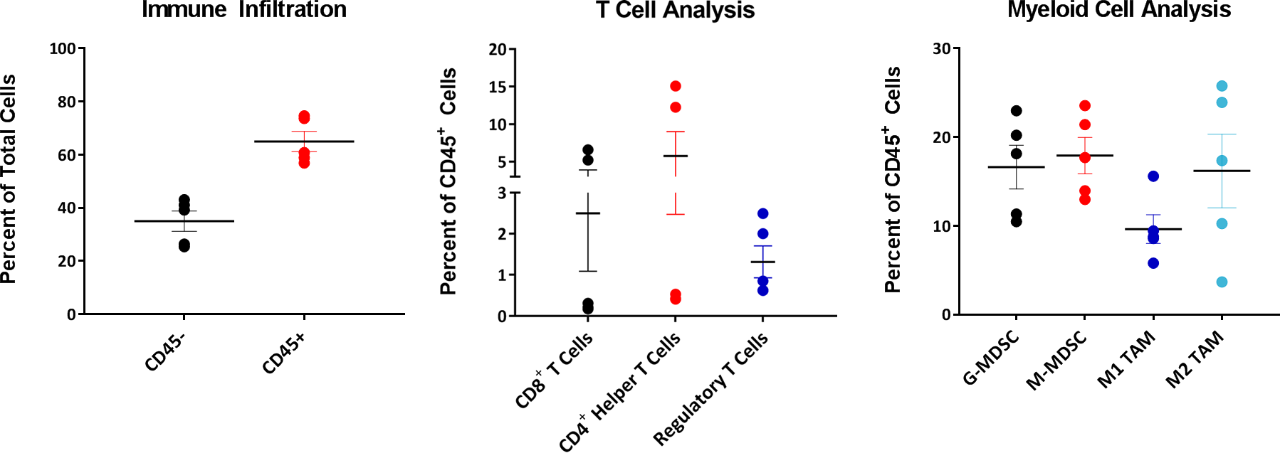
Fig 4: EMT-6 Infiltrated T cells and Myeloid Cell Analysis
By contrast, the T cell compartment demonstrated intertumor variability with three of five tumors showing very low levels of CD4+ and CD8+ T cells. Interestingly, the Treg composition in these tumors exhibited much less variability between tumors when compared to CD4+ and CD8+ T cell infiltrates. Studies are ongoing to determine whether the variability seen with infiltrated CD4+ and CD8+ T cells across these EMT-6 tumors is inherent to the biology of this model or if variability would normalize given a larger sample size.
EMT-6 – A Robust Preclinical Immuno-Oncology Model
The EMT-6 syngeneic breast carcinoma model has a favorable immune profile and can be employed as a robust preclinical immuno-oncology model. Our data supports the use of this tool in investigating novel treatment combinations with radiation, checkpoint inhibitors, or costimulatory molecules.
MODEL SPOTLIGHT | EMT-6 Syngeneic Breast Tumor Model – A Powerful Tool for Immuno-Oncology Studies (PDF Version)
